Arrhenius Kinetics nader makarious
Biography
Svante August Arrhenius was born on February 19, 1859,
the son of Svante Gustaf Arrhenius and Carolina Christina Thunberg. His
ancestors were farmers; his uncle became Professor of Botany  and Rector
of the Agricultural High School at Ultuna near Uppsala and later Secretary of
The Swedish Academy of Agriculture. His father was a land surveyor employed by
the University of Uppsala and in charge of its estates at Vik, where Svante was
born. The family moved to Uppsala in 1860. The boy was educated at the
Cathedral school where the rector was a good physics teacher. From an early age
Svante had shown an aptitude for arithmetical calculations, and at school he
was greatly interested in mathematics and physics. In 1876 he entered the
University of Uppsala, studying mathematics, chemistry and physics. The
practical instruction in physics was not of the best, and in 1881 he went to
Stockholm to work under Professor E. Edlund at the Academy of Sciences.
and Rector
of the Agricultural High School at Ultuna near Uppsala and later Secretary of
The Swedish Academy of Agriculture. His father was a land surveyor employed by
the University of Uppsala and in charge of its estates at Vik, where Svante was
born. The family moved to Uppsala in 1860. The boy was educated at the
Cathedral school where the rector was a good physics teacher. From an early age
Svante had shown an aptitude for arithmetical calculations, and at school he
was greatly interested in mathematics and physics. In 1876 he entered the
University of Uppsala, studying mathematics, chemistry and physics. The
practical instruction in physics was not of the best, and in 1881 he went to
Stockholm to work under Professor E. Edlund at the Academy of Sciences.
http://nobelprize.org/nobel_prizes/chemistry/laureates/1903/arrhenius-bio.html
Arrhenius equatoin
k=Ae(-Ea/RT)
ln(K) = ln Ae(-Ea/RT)
=
ln A + ln e(-Ea/RT)
ln (K) = ln(A)- (Ea/R) (1/T)
ln (K) = (-Ea/R) (1/T) + ln A
from the given data at
http://web.lemoyne.edu/~giunta/classicalcs/arrkin.html
microsoft excel will be used to graph this date twice as
below
First graph
Shows the relation between rate of the reaction and
temprutre in Kelvin
Second graph
Will apply Arrhenius equation and graph the relatoin
between 1/T in Kelvin and ln(K).
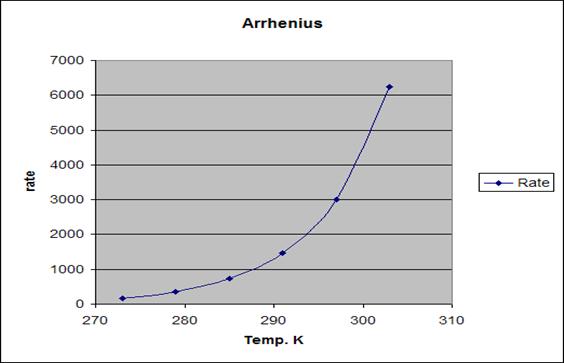
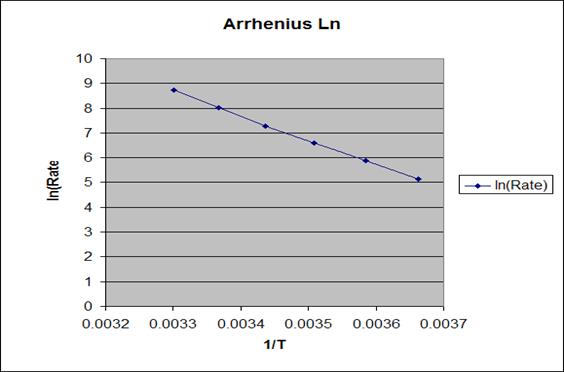
The first graph shows certain kind of relation,but doesn’t
give enough information neither about the reaction nor the activation engery Ea
which is very important in all chemical reactions.
The second graph (using Arrheius equation) shows a direct
and inverse relation between ln (K) and (1/T), also from the graph we can
figure out the activation energy Ea buy taking the slop (Y1-Y2/X2-X1)